Enthalpy Change of Atomisation
That will be the same irrespective of which halogen you are talking about. B Calculate the bond energy of C-H bond given that the heat of formation of CH 4.
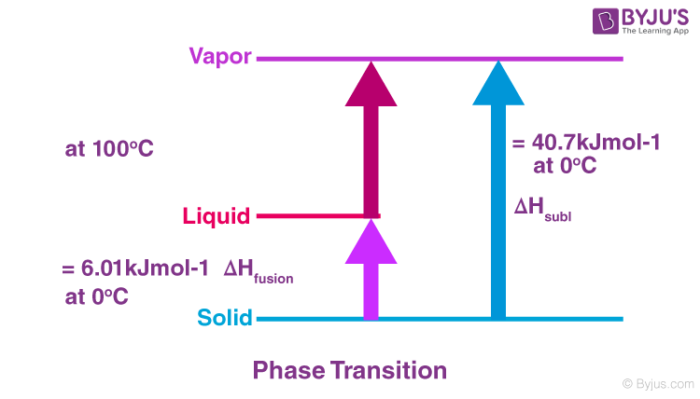
Calculate the enthalpy change on freezing of 10 mol of water at 100C to ice at 100C.

. Visit BYJUS to understand the properties structure and uses of Cellulose C6H10O5n explained by Indias best teachers. Enthalpy of atomization Δ a H 0 is the change in enthalpy when one mole of bonds is completely broken to obtain atoms in the gas phase. Chemical Engineering Journal has been ranked 3 over 336 related journals in the Industrial and Manufacturing Engineering research category.
Cellulose C6H10O5n - Cellulose is the chemical name of C6H10O5n. CH 4 a H 0 16650 kJ mol-1. Heat will be given out when the changes involving the sulphuric acid occur.
Moreover q is equal to the standard enthalpy change only when the reactants and products are both at the same temperature normally 25C. Thus chemical reactions can occur faster and the reactivity increases down the group. Amount of energy required to dissociate 1 mole of the stable molecule into a gaseous atom known as the heat of atomisation.
The equilibrium constant denoted as k is a number. When equilibrium concentrations are represented in atmospheric pressure Kp is the equilibrium constant to use and when equilibrium concentrations are expressed in molarity Kc is the equilibrium constant that should be used. Why is it called enthalpy of atomisation.
Atomization of methane molecule. For diatomic molecules the enthalpy of atomization is equal to the enthalpy of bond. This isnt the total enthalpy change for the whole reaction.
Kp and Kc are the equilibrium constants for an ideal gaseous mixture. A H 603 KJ mot1 at 0C. This quantity decreases going down the group and so does the activation energy.
Adding the atomisation and first ionisation energies gives a quantity closely related to but not equal to the activation energy of the reaction of an alkali metal with another substance. The Heat of Atomisation or Heat of Atomisation. Journals Impact IF Ranking In the Industrial and Manufacturing Engineering research field the Quartile of Chemical Engineering Journal is Q1.
The total enthalpy change will be the sum of the enthalpy changes for the halide ion half-reaction and the sulphuric acid half-reaction. The ranking percentile of Chemical Engineering Journal is around 99 in.
Enthalpy Of Atomisation Atomisation Of Transition Elements

Caie 9701 11 O N 20 Q 6 Enthalpy Changes Youtube

A Level Bond Enthalpy Bond Dissociation Energy Calculations For Enthalpy Of Reaction Ks5 Gce Chemistry Revision Notes
No comments for "Enthalpy Change of Atomisation"
Post a Comment